The role of heated, humidified resuscitation in reducing pre-term hypothermia

The landscape of neonatal care has changed drastically since premature babies were cared for in tiny glass cabinets by Dr Martin Couney’s nurses on Coney Island, New York, USA. Many simple and other novel techniques and therapies have been introduced and implemented over the years as we now care for babies that are smaller and more fragile than ever before.
The increasingly complex set of extremely preterm infants bring with them their own set of challenges, not least the difficulty of keeping them warm. Thermoregulation and prevention of hypothermia is already high on the list of priorities in maternity departments delivering healthy term babies, however, in the same environment where the very low birth weight (VLBW) infant is exceedingly more fragile and affected by heat loss, other techniques may need to be adopted to prevent dangerous decreases in core body temperature.
International recommendations require the core body temperature of a newborn be kept within a range of 36.5°C – 37.5°C to provide the best outcomes for that baby [1] and neonatal units record the temperature of babies upon admission as a marker of quality of care and predicted patient outcome. Studies have shown that even as little as a 1°C drop in admission temperature increases the risk of sepsis in a VLBW newborn by 11% with the overall risk of morbidity increasing by 28% [2,3]. Couple these statistics with the evidence that body temperature can fall by as much as 4°C in the first 10-20 minutes of life, if the newborn infant is left without thermal protection [4], the risk and associated outcomes of hypothermia to VLBW infants is profound.
Image 1: GE Giraffe Warmer (Giraffe Warmer | GE Healthcare (United States)
Image 2: Vygon Neohelp TM ( Vygon Neohelp Heat Loss Bag)
Various equipment is available to provide a positive impact in situations where babies get cold easily [2], (Image 1 & 2). This blog will specifically focus on the delivery of warmed gases when providing respiratory support to the VLBW baby, which is a requirement in many preterm deliveries.
Clinical trials, including the 2014 study by Meyer et al. [5], have shown the benefits of using warmed gases when stabilising VLBW infants and providing Positive Pressure Ventilation (PPV). The concept is not alien, after all, it is widely accepted and practiced that any therapeutic and supportive acute respiratory therapies delivered in the neonatal ward setting are done so with active heat and humidification.
Current practice in many countries see babies requiring respiratory support after birth given this on a platform bed, (e.g., Image 2 above) and via a t-piece resuscitation device built into the upright manifold extending from the back of the bed. This allows for the support required to be initiated almost instantly for the 10% of babies that do not breathe when they are born [6], however, the gases delivered through this PPV system come directly from a piped gas source or gas cylinders and as a result can be described as ‘cold and dry’. In fact, an investigation carried out across different areas in one hospital demonstrated piped oxygen having a mean temperature of 23.3°C and a Relative Humidity (RH) of only 2.1%, while piped air was measured at 23.4°C with a RH of 5.4% [7], which are rather stark results.
With the already fragile VLBW baby receiving PPV and stabilisation with these cold and dry gases it follows a simple train of thought that this could lead to the infant’s body temperature lowering outside of the recommended range. At the very least we can expect that given the massive impact of conduction, convection, radiation, and evaporation [8] on these premature babies, the cold and dry gases are not useful in combating the heat loss associated with these thermodynamic forces and the use of warmed and humidified gases would assist with this.
Key also to the use of heated and humidified gases is the benefit it provides to enhanced lung function.A meta-analysis of studies including a total of 476 preterm infants <32 weeks’ gestation showed a reduction in admission hypothermia by 36% supporting their recommendation to consider heating and humidification of inspired gases during stabilisation after birth and also during transport to the neonatal unit [9].
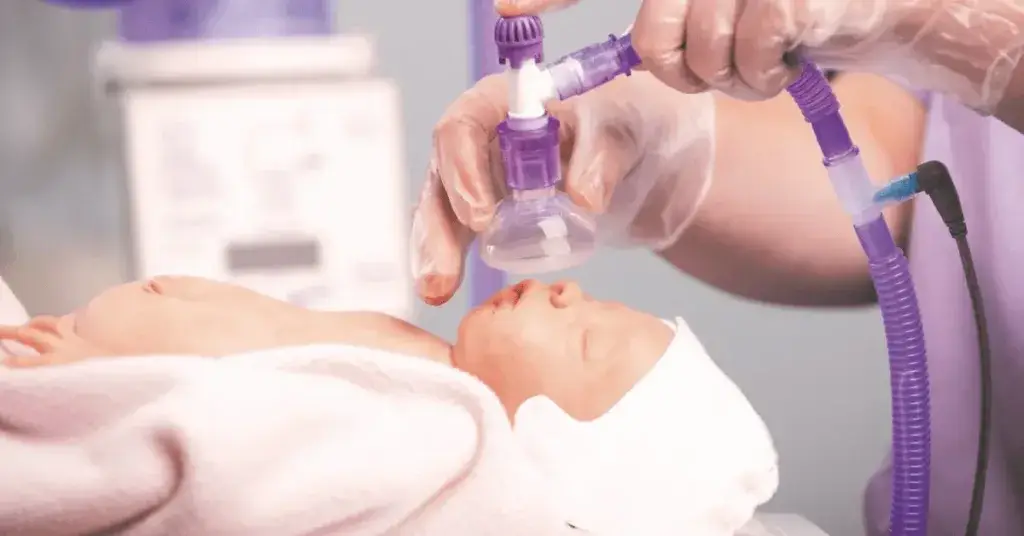
The PPV system is easily converted to a heated, humidified version of the same by choosing an alternative circuitry type to the simple tubing limb and adjustable pressure patient elbow that is supplied with standard circuitry options. A heated, humidified t-piece circuit includes a heated wire limb and humidification chamber which both work in conjunction with an electrical heater humidifier supplying a heat source and heater wire power to add heat and water vapour to the system as gas passes through the humidification chamber. This type of system is a familiar sight in neonatal intensive care units globally and as a consequence may therefore reduce the need for additional training. Perhaps, given the fact that such an easily adopted solution is readily available the practice and policy change, as well as convincing those reluctant to adapt, may not be the challenge that is anticipated.
With the very real risk of hypothermia presenting itself in all settings where preterm babies are born, we are fortunate to have such an array of options available to provide a positive outcome. While the implementation of just one such practice change could improve admission temperature, exploring all options and the potential to provide multiple beneficial interventions may have a worthwhile effect on the body temperature of these vulnerable infants.
The transition to a newborn is the most complex physiologic adaptation that occurs in human experience [10] and all too often there is a need to help assist the newborn with this transition. As the end goal of any respiratory therapy is to replicate a healthy human body, with adequate respiratory effort, it seems logical to warm the gases to assist with this process.