Reducing Medical Device Related Pressure Ulcers

Hospital patients susceptible to pressure ulcers during a stay in hospital is both unfortunate and an expensive burden to any healthcare system. Whether the ulcers are a result of an actual physical illness or secondary to a medical device that has been used to treat the illness, the burden of pressure injuries in hospital patients remains substantial with over one in ten adult patients admitted to hospitals affected. [1]
The term ‘pressure ulcer’ is generally used in the US, the UK tend to use the term ‘pressure sores’ but now the classification seems to be more separated between pressure ulcers and medical Device-Related Pressure Ulcers (DRPU). Certain patients tend to be more susceptible to a pressure ulcer such as those who are frail, malnourished, oedematous, elderly or may have diabetes, heart disease and are most of all immobilised. The DRPU patient, without any co morbidities, can be at risk simply by having medical devices placed on or in them because the medical device causes focal and localised forces that deform the superficial and deep underlying tissues. [2]
Whatever the initial cause of the pressure ulcer, a potential increased length of stay, increased nursing time, added pain management treatment, wound management leading to increased monetary costs are just one aspect, but the psychological impact ulcers have on patients cannot be ignored; less sleep, more pain and the impact on family members, being just a few consequences.
A variety of medical devices have been shown to increase the risk, with patients 2.4 times more likely to develop a pressure ulcer if any medical device is used. [3] To put that into context, 10-35% of hospital acquired pressure ulcers are directly related to medical devices. [4]
According to the NOECCN (North of England Critical Care Network) examples of devices associated with DRPU include: endotracheal tubes, orthotic devices, bed frames, spectacles and continuous positive airway pressure (CPAP) masks. [5]
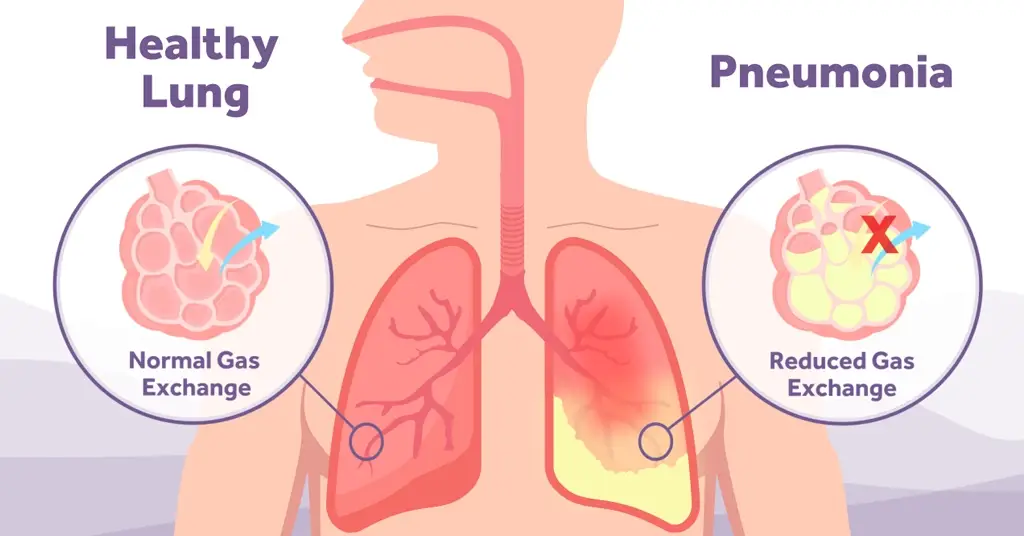
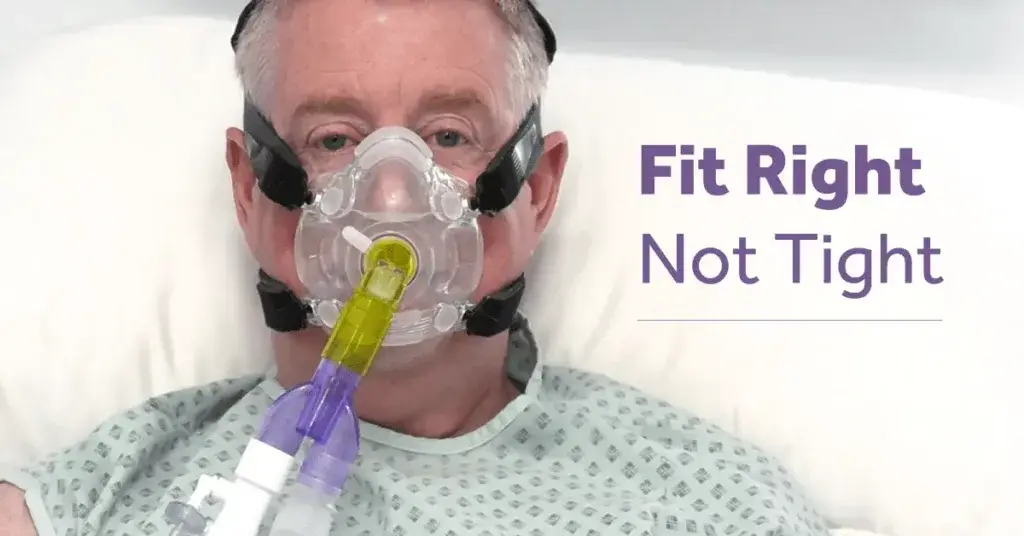
Facial DRPUs caused by CPAP masks appear among 10% to 33% of CPAP users within several hours of application of the mask; these injuries are associated with the sustained soft tissue deformations caused by mounting and tightening the mask to the head [6], and the extreme microclimate conditions that expose the facial skin under and near the mask to almost 100% humidity.[5] As the mask directly compresses and shears the heated facial skin and underlying soft tissues against the rigid skull surface (and more so when the straps are overtightened), extreme mechanical stress concentrations develop in the affected tissues.
The development of pressure ulcers can result in intolerance to the treatment and potentially failure of the treatment. Patient comfort and enhanced compliance are key factors in determining patient outcome.
A DRPU on the bridge of the nose, where the tissue has no padding, may rapidly progress from category I to category IV or unstageable.
Factors that increase the risk of DRPU include:
- the patient’s inability to sense the device and the associated pressure, friction and shear on their skin due to sedation
- the patient’s inability to reposition themselves duration of use
- duration of use
- the perceived need to secure a device tightly to ensure correct function.
The consensus, set up in 2019, puts an onus on healthcare companies to increasingly offer education and training on their products; it is vital this includes DRPU prevention. Manufactures should also include instructions for use and clear and detailed information on:
- Instructions on application, fitting and securement
- Instructions on how to continuously monitor and adjust the device
- Information on the presence of interface materials and structures within the device that have been shown to be effective in preventing DRPU (supporting published bioengineering and clinical evidence on their efficacy should be cited). [5]
As we know, during the global pandemic, CPAP was a growing treatment used within various areas of hospital settings and recent reports of the effect of the COVID-19 virus on the epidemiology of pressure ulcers in general, and DRPUs in particular, demonstrate a sharp rise in incidence and prevalence. For example, in the UK, the overall pressure ulcer rate per 1000 beds in acute care increased from a pre pandemic level of around 1 to over 2.7 in the first month of the pandemic. This rise is specifically associated with the increase in DRPUs and prone positioning in the expanded critical care patient population. [7]
With improved awareness and synergy between the manufacturers and health care professional fitting the masks, we will hopefully start to see a decline in DRPUs caused by CPAP and NIV masks and cost burdens to the health system can reduce in time.
[3] Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Int Wound J. 2010;7((5)):358–65)
[5] Device-related pressure ulcers: SECURE prevention (noeccn.org.uk)
[6] Wounds UK, Vol 17, No 3; 2021, REVIEW 32 Medical device-related pressure ulcers and the covid-19 pandemic: From aetiology to prevention | ResearchGate
[7] Medical Device-Related Pressure Injuries During the COVID-19 Pandemic – PubMed (nih.gov)